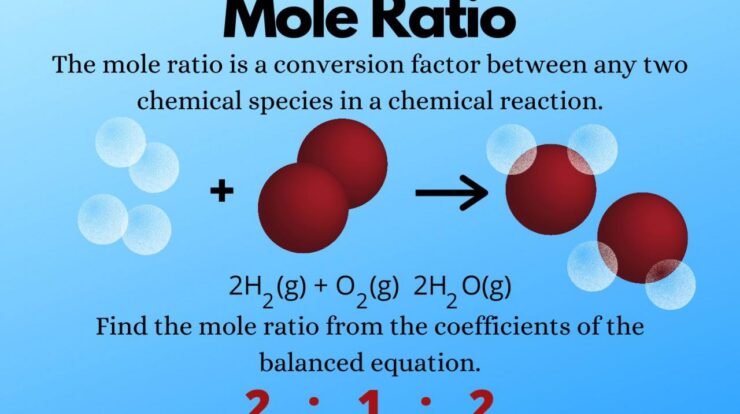
Mole measurement, a fundamental concept in chemistry, unravels the secrets of quantifying substances with precision. It establishes a universal language for expressing the amount of matter, enabling scientists to decipher the intricate dance of chemical reactions and unravel the mysteries of the molecular world.
This in-depth exploration will delve into the significance of mole measurement, unraveling the methods employed to determine it, and showcasing its diverse applications across scientific disciplines.
Mole Measurement: A Fundamental Unit in Chemistry
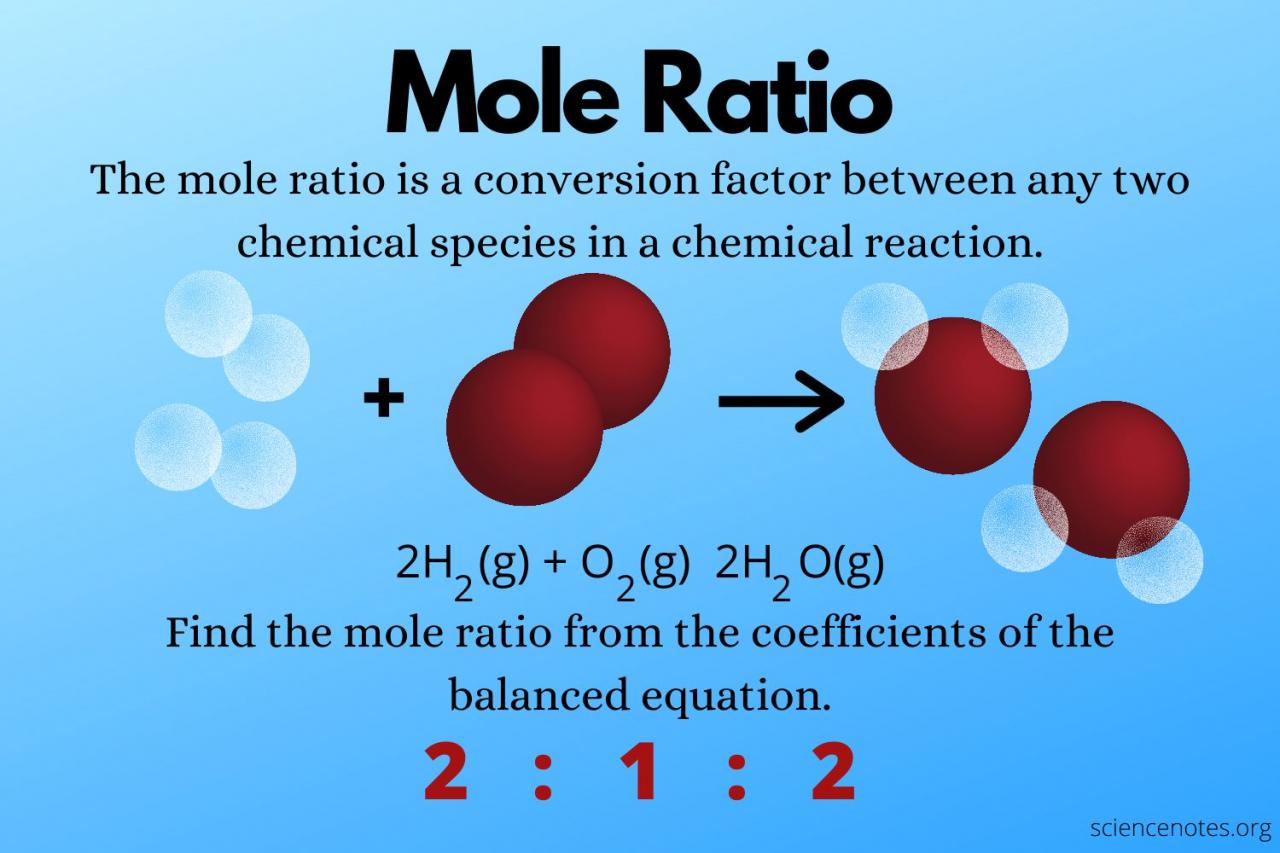
In the realm of chemistry, the concept of a mole serves as a cornerstone for understanding and quantifying the composition and behavior of substances. This unit of measurement plays a crucial role in determining the amount of a substance present, facilitating precise calculations and ensuring accurate analysis in various scientific fields.
Definition and Importance of Mole Measurement
A mole, denoted by the symbol “mol,” is defined as the amount of a substance that contains exactly 6.02214076 × 10^23 elementary entities. These entities can be atoms, molecules, ions, or electrons, depending on the context. The mole concept provides a standardized way to express the quantity of a substance, allowing scientists to compare and relate different substances based on their molar mass.
The significance of mole measurement lies in its ability to determine the precise amount of a substance involved in a chemical reaction or present in a given sample. By knowing the number of moles of a substance, scientists can calculate its mass, volume, and other properties with remarkable accuracy.
Methods for Determining Mole Measurement
There are two primary methods for determining the mole measurement of a substance: gravimetric analysis and volumetric analysis.
Gravimetric Method
The gravimetric method involves measuring the mass of a substance. A known mass of the substance is carefully weighed and then converted to moles using its molar mass. This method is particularly useful for solids and liquids with well-defined compositions.
Volumetric Method
The volumetric method involves measuring the volume of a substance. A known volume of the substance is accurately measured and then converted to moles using its molarity or concentration. This method is commonly used for solutions and gases.
Applications of Mole Measurement
Mole measurement finds widespread applications in various scientific fields, including chemistry, biochemistry, and environmental science.
Stoichiometric Calculations
In chemistry, mole measurement is essential for performing stoichiometric calculations, which involve determining the quantitative relationships between reactants and products in a chemical reaction. By knowing the number of moles of each reactant and product, scientists can predict the amount of reactants required or the amount of products formed.
Limiting Reactant Determination
Mole measurement also helps determine the limiting reactant in a chemical reaction. The limiting reactant is the reactant that is completely consumed during the reaction, thereby limiting the amount of product that can be formed. Identifying the limiting reactant is crucial for predicting the maximum yield of a reaction.
Applications in Various Fields
Beyond chemistry, mole measurement has applications in fields such as biochemistry, environmental science, and materials science. In biochemistry, it is used to determine the concentration of proteins and other biomolecules. In environmental science, it is used to measure the concentration of pollutants in air and water.
In materials science, it is used to characterize the composition and properties of materials.
Common Mistakes and Misconceptions, Mole measurement
While mole measurement is a fundamental concept in chemistry, there are some common mistakes and misconceptions that can arise.
Mistakes in Calculations
One common mistake is making errors in mole calculations, such as using incorrect molar masses or converting between units incorrectly. It is important to double-check calculations and ensure accuracy.
Misconceptions about the Mole
Another misconception is that a mole of all substances has the same mass. While the number of entities in a mole is the same for all substances, the mass of a mole varies depending on the molar mass of the substance.
Tips for Avoiding Errors
To avoid errors in mole measurement, it is essential to understand the concept thoroughly, practice mole calculations regularly, and double-check results. Additionally, using reliable sources of information and seeking guidance from experts can help prevent misconceptions.
Summary
Mole measurement stands as a cornerstone of chemistry, providing a precise and versatile tool for understanding the composition and behavior of matter. Its applications extend far beyond the laboratory, shaping our understanding of the world around us and driving advancements in fields as diverse as medicine, materials science, and environmental chemistry.
FAQ Overview: Mole Measurement
What is the definition of a mole?
A mole is the SI unit of amount of substance, defined as the quantity of matter containing as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
How is mole measurement used in stoichiometric calculations?
Mole measurement allows us to determine the exact amounts of reactants and products involved in a chemical reaction, enabling precise predictions of reaction outcomes.
What are the advantages of the gravimetric method for determining mole measurement?
The gravimetric method offers high accuracy and precision, making it suitable for precise quantitative analysis.